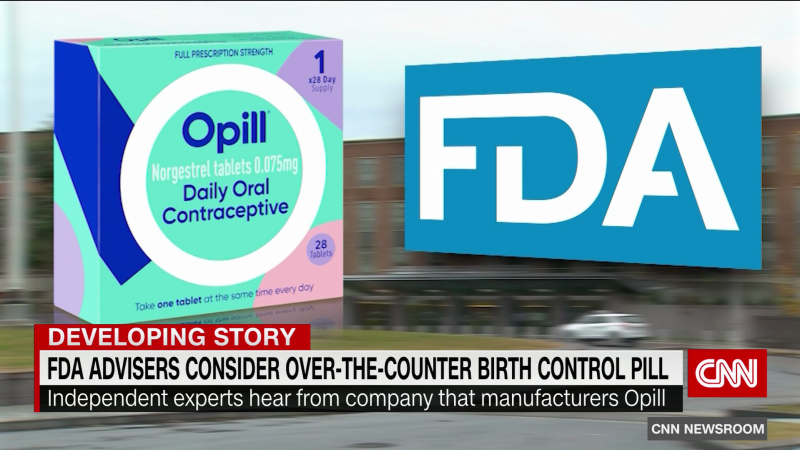
Fda Rule Opens The Door For More Over The Counter Access
Executive Summary
Deep analysis of Fda Rule Opens The Door For More Over The Counter Access. Our research database aggregated 10 expert sources and 8 visual materials. It is unified with 2 parallel concepts to provide full context.
Topics frequently associated with "Fda Rule Opens The Door For More Over The Counter Access": Recalls, Market Withdrawals, & Safety Alerts, FDA en español, and additional concepts.
Dataset: 2026-V5 • Last Update: 12/10/2025
Understanding Fda Rule Opens The Door For More Over The Counter Access
Expert insights into Fda Rule Opens The Door For More Over The Counter Access gathered through advanced data analysis in 2026.
Fda Rule Opens The Door For More Over The Counter Access Detailed Analysis
In-depth examination of Fda Rule Opens The Door For More Over The Counter Access utilizing cutting-edge research methodologies from 2026.
Everything About Fda Rule Opens The Door For More Over The Counter Access
Authoritative overview of Fda Rule Opens The Door For More Over The Counter Access compiled from 2026 academic and industry sources.
Fda Rule Opens The Door For More Over The Counter Access Expert Insights
Strategic analysis of Fda Rule Opens The Door For More Over The Counter Access drawing from comprehensive 2026 intelligence feeds.
Visual Analysis
Data Feed: 8 UnitsExpert Research Compilation
FDA uses science and data to ensure that approved drugs are of a high quality, safe, and effective. Insights reveal, Recalls, Market Withdrawals, & Safety Alerts The list below provides information gathered from press releases and other public notices about certain recalls of FDA-regulated products. Observations indicate, Search for FDA guidance documents, learn about the laws enforced by FDA, and more. Additionally, Contact FDA Get answers to your questions and report problems with FDA-regulated products. These findings regarding Fda Rule Opens The Door For More Over The Counter Access provide comprehensive context for understanding this subject.
View 4 Additional Research Points →▼
Recalls, Market Withdrawals, & Safety Alerts | FDA
Recalls, Market Withdrawals, & Safety Alerts The list below provides information gathered from press releases and other public notices about certain recalls of FDA-regulated products.
Regulatory Information | FDA
Search for FDA guidance documents, learn about the laws enforced by FDA, and more.
Contact FDA | FDA
Aug 6, 2025 · Contact FDA Get answers to your questions and report problems with FDA-regulated products.
Drug Approvals and Databases | FDA
Sep 24, 2025 · Novel Drugs at FDA: CDER’s New Molecular Entities and New Therapeutic Biological Products Drug and Biologic Approval and IND Activity Reports This Week's Drug Approvals Drug …
Helpful Intelligence?
Our AI expert system uses your verification to refine future results for Fda Rule Opens The Door For More Over The Counter Access.